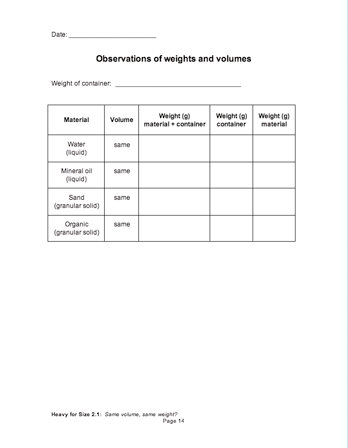
Same volume, same weight?
Plan Investigation 2.1

Imagine three identical buckets: one filled with water, one filled with shredded leaves and twigs, and one filled with sand. Each material takes up the same amount of space, but do they weigh the same? It’s an old conundrum, and one that will take students four investigations to unravel completely.
In this investigation, students share what they know about volume, then compare the weights of equal volumes of two liquids and two granular solids using a digital scale. By the end of the investigation students will understand that volume describes the amount of space something takes up, that equal volumes of different materials can have different weights, and that some materials are "heavy for their size."
Learning Goals
- Distinguish weight and volume
- Learn how to use a digital scale
- Understand that "heavy for size" is property of materials
| Sequence of experiences | ||
|---|---|---|
| 1. Ask the question | All Class | 10 Mins |
| 2. Compare the weights | Small Groups | 20 Mins |
| 3. Make meaning | Discussion | 15 Mins |
Materials and Preparation
For the class:

- Post the investigation question in a place where all students can see it.
- Make a class data table for recording the weights of the four samples; an example is found in Step 2.
- 1 unopened ream of 8.5 x 11 in. paper (not in kit)
- 1 150cc container approximately one–quarter filled with fresh water
- 1 150cc container approximately three–quarters filled with fresh water
- 1 150cc container filled to the top with sand
- 1 150cc container filled to the top with organic soil
- 1 20oz cup
For each tray:

- 1 capped 150cc container containing exactly 40cc of fresh water (from Investigation 1.1)*
- 1 capped 150cc container containing exactly 40cc of mineral oil (from Investigation 1.1)*
- 1 capped 150cc container containing exactly 40cc of sand*
- 1 capped 150cc container containing exactly 40cc of organic soil*
- 1 capped 150cc container, empty (to establish tare weight)
- 1 digital scale
* Note: These 4 containers are used again in the next investigation. It is key to this investigation that these four containers holding water, mineral oil, sand, and organic soil all have volumes that are close as possible to 40 cc's. All should appear to hold the same volume. Tap the containers holding sand and organic soil to settle the material in those two containers.
1. Ask the question
Review the concept of volume
Get students talking about volume.
When you hear the word ‘volume’ what do you think about?
Other ideas about volume: When they first hear the word "volume," many students will think about the loudness of sound, or about volumes of books. Some students may offer a formula for calculating volume, e.g. height x width x length.
Acknowledge that the term volume has different meanings, and that knowing how to calculate volume is important. Explain that at this time we're talking about volume as the amount of 3-dimensional space that an object takes up.
Students typically confuse volume with measurements such as area or perimeter. Ask students explicitly to contrast area and volume. How are they different.
The brief demonstration below will help establish the difference between area and volume or 3-D space.
1. Show the class an unopened ream of 8.5 in. x 11 in. paper and ask students to identify some objects that have a volume that is less than the ream of paper - objects that take up less 3-dimensional space than the ream of paper. Their responses can help you gain insight into their grasp of the concept of volume.
2. Ask students to identify objects that have a volume greater than the ream of paper.
Note: Students may identify objects made of two or more materials, such as a container filled with a soft drink, or an object that includes air, such as a basketball. Those are acceptable examples, as long as you clarify that the volume of the object includes the volume of the aluminum and the volume of the soda, or the volume of the leather basketball and the volume of the air that is inside of it.
- How many of these reams of paper can I fit into my pocket?
- None. The volume of my pocket is less than the volume of the ream.
- How many of these reams can you fit into a backpack?
- Two? Three? Four?
3. Place the ream of paper flat down on a desk that all students can see, and point out that the ream is now covering part of the surface area of the desk. The ream still has its volume. That has not changed.
4. Rotate the ream so that the 2-3/4 in. x 11 in. side is on the desk surface.
- Is the ream of paper now covering more or less of the desk's surface area?
- Less
- Has the volume of the ream of paper increased, stayed the same, or decreased?
5. Summarize: Volume refers to 3-dimensional space; surface area refers to flat, 2-dimensional space. The flat top of the desk has a surface area. Each of the six faces of the ream of paper has a surface area. The ream of paper can cover different amounts of the desk's surface, depending on how the ream is placed on the desk. The amount of 3-dimensional space that the ream takes up remains the same, no matter which way we turn it or how much of the desk's surface area the ream covers.

Show the two containers that hold different volumes of water.
- How do the volumes of water in these two containers compare?
Show the container filled with sand and the container filled with organic soil.
How do the volumes of sand and organic soil compare?
Which one weighs more? How can we tell?
Introduce the investigation question
Today's investigation question is:
Same volume, same weight?
Show the class two containers, one holding 40 cc's of sand and one holding 40 cc's of organic soil.
- What do you think? If samples of different materials have the same volume, do they have the same weight?
2. Compare the weights

Introduce the electronic scales
Distribute the electronic scales that students will be using in this unit. Explain that that scales have been made specifically for weighing objects that are light. They could weigh a couple of apples, but not a brick. The class will be using just light objects in the science unit. Students should handle the scales carefully and not press down on them or place any heavy objects on them.
Letter from the Engineer
A better way to weigh
This scale you are about to use looks very different than the pan balances you may have used in the past to weigh things. Do you remember how to weigh things using a double pan balance? You place an object you want to weigh in one pan and add weights to the pan on the opposite side until the two pans are level.
Engineers know that a pan balance is difficult to use. Sometimes the weight you add is too heavy or too light and nothing seems to make the pans balance perfectly. Sometimes the weight you need can get lost. And even when you can balance the pans perfectly, it can take a lot of time. Engineers saw these problems and asked, “How can we improve this instrument? Can we make a scale that is easier and faster to use, is accurate, and that does not need a set of weights to balance an object. One solution they came up with is the electronic scale, which is the kind of scale you are about to use.
How does the electronic scale work? It depends on the fact that placing weight on something causes it to bend or change its shape. Sometimes you can see this very clearly: Think about a diving board. Once someone walks out to the end of the diving board, it bends to a new position. The change in shape or position is called a “deflection”. After the person leaves the diving board it returns to its original position and the deflection that was caused by the weight goes away.
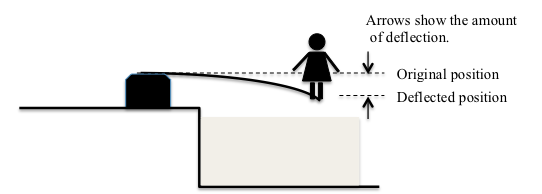
Even when the weight is very small, deflection happens, although it can be too small for your eyes to notice. If a small bird landed on the end of the diving board, the board would deflect a very tiny amount but no one would notice it.
When we place an object on the top of an electronic scale, we are causing a small piece of metal inside the scale — you can think of it as a tiny diving board — to bend or deflect; the heavier the object, the more the piece of metal deflects. Engineers had to study how different materials deflect or bend when weight is put on them; some deflect much more than others. They have designed a way to measure that deflection, and to have batteries send a certain amount of electricity to the LCD (screen that shows the weight) depending on how much the metal deflects. More deflection means more weight. Both the pan balance and the electronic scale are examples of technology that help us to weigh things, and the electronic scale is a good example of how engineers have developed a new technology to solve a problem.
Demonstrate how to turn the scale on and have students turn on their scales.
Some scales can be set to measure either in grams or in ounces. For all work in this unit, students should check the units each time before they start to use the scale, and use the switch or button to reset it to grams if that is necessary.
Finally, a scale will sometimes indicate a weight, or even a minus number, when nothing is on it. This would result in an incorrect measurement. Show students how to reset the scale to zero; they should always be sure the scale reads zero before they weight anything.
Have students place a pencil or other small object on the scale and observe the way in which the scale displays the weight.
Compare two liquids
Distribute a tray of materials to each group and identify the four earth materials: water, mineral oil, organic soil, and sand.
Hold up the containers of water and mineral oil and confirm that they hold the same volume of liquid. It is not necessary to measure the volumes, just ask the class to compare them "by eye."
Before you weigh the two samples, make a prediction: How do you think the weights compare?
Listen carefully to the reasoning. Do students think the weights will be the same “because the volumes are the same”? Do they think they‘ll weigh the same “because both materials are liquids”? Or maybe they think the weights will be different because the liquids have different properties, perhaps “because one liquid is thick and the other is thin.” Acknowledge all answers and theories.
- How can we find out for sure?
- By weighing the samples
If students don't raise the question themselves, ask how they can measure the weight of only the liquid
How can we weigh just the water, or just the mineral oil?
Listen for a suggestion to subtract the weight of the empty container from the total weight of the sample. Introduce the term tare weight, which is the weight of just the container.
Have groups weigh an empty container and record that weight (approx. 26g) in all four rows of the data table their Science Notebooks [Observations of weights and volumes]. Next have them weigh the two liquid samples, calculate the weight of just the liquids, and enter their data in their notebooks. Make sure they specify that the unit of weight is grams.
When all the groups are finished, ask for their results and record them in the table you prepared before class. Do not try to reconcile differences at this point.
Example of a class data table.
| Material | Volume or Bulk Volume |
Weight (g) Material |
Class Agreement Weight (g) |
|---|---|---|---|
| Water (liquid) |
40cc | 40g; 40g; 39; 40g; 41g; 39g | |
| Mineral Oil (liquid) |
40cc | 32g; 31g; 31g; 32g; 33g; 33g | |
| Sand (granular solid) |
40cc | 78g; 76g; 77g; 77g; 78g; 78g | |
| Organic (granular solid) |
40cc | 17g; 16g; 15g; 16g; 16g; 20g |
Compare two solids
Have students pass around the containers of sand and organic soil.
- Do the samples seem to have the same volume?
- Do you think they have the same weight?
Have students weigh the samples as before, subtracting the tare weight and recording the data in their notebooks. Make sure they label their measurements in grams.
The procedure is exactly the same as before, but this time there’s a wrinkle — there are empty spaces between the grains of soil.
- Are the empty spaces parts of the volume?
Yes. Sand and organic soil belong to a group of materials called granular materials. Cheerios, sugar, gravel, and peanuts are also granular materials. Granular materials are made up of many smaller pieces that are about the same size and that have spaces in between them. The volume of a granular material is the sum of two smaller volumes: 1) the volume of all the individual pieces, and 2) the volume of all the small spaces. This combined volume has a special name: it is called bulk volume. Bulk volume is measured the same way that the volume of a liquid is measured, but the name bulk volume lets everyone know that the volume is a combination of the individual pieces and the spaces between them.
Once students have weighed the samples and entered the data in their notebooks, enter their data in the class table.
Allow time for students to briefly discuss the reflection questions in their small groups and write responses in their notebooks before moving on to the class discussion [Reflections on weights and volumes].
3. Make meaning
There are two layers of data to grapple with in this discussion:
(1) establishing a single weight for each of the four samples, since it's unlikely that all groups found identical weights for a sample;
(2) once the weight of a 40cc sample is established for each material, using the data to address the investigation question: Same volume, same weight?
Purpose of the discussion
The purpose of the discussion is for students to
- connect the investigation question and their data.
- wrestle with discrepant data.
- make claims and describe the supporting evidence.
- suggest explanations.
Again, the discussion focuses on the investigation question.
Engage students in the focus question
Remind students that the purpose of collecting the weight data was to answer the investigation question:
Same volume, same weight? If samples of different materials have the same volume, do they have the same weight?
Do we have data that we can use to answer this question?
As you listen to student responses, check to be sure everyone knows what information is contained in each of the three columns in the class data table. The class table is a simplified version of the one in their notebooks.
How can we reconcile discrepancies in the data?
Not all groups found the same weight for 40cc of water (oil, sand, organic soil). Why do you think that is and what number should we use for the weight?
Listen to students‘ ideas about why their weight measurements vary and how they can decide on a "class" value for the weight of 40cc of water.
Some possible student ideas
Explanations for small differences
- There may be tiny differences in the amount of sand (water, oil, organic soil) in the containers.
- There may be tiny differences between the scales.
- The scales round the actual weight to the nearest gram, so tiny differences in actual weight can appear to be larger on the scales.
Explanations for large discrepancies
- We forgot to include the weight of the container cap.
- We made a subtraction error.
Strategies for coming up with a weight the class can agree on
- Use the weight that appears most often.
- Pick a round number that seems an appropriate middle point.
- Make a new 40cc sample and weigh it.
Once a typical weight for each material has been established, ask someone to make up a sentence that describes the data in each row of the table. For example:
The top row tells us that a forty cubic centimeter sample of water weighs forty grams.
What claims can we make and what is the supporting evidence (data)?
Now that the data has been "cleaned up", ask:
- Let's return to the investigation question: Same volume, same weight? If samples of different materials have the same volume, do they have the same weight?
The cleaned-up data should be clear: equal volumes of different materials can have different weights. Encourage students to describe the data they use as evidence for a claim.
What is a possible explanation?
Ask the group to explain why they think same-sized samples of earth materials have different weights.
- What might be a possible explanation for why, when the volumes are the same, the weights of different materials are different?
Listen to students‘ ideas. If they don't suggest that each kind of material has its own properties, you might remind them of their prior experience with properties of materials. In addition to properties such as color or texture, materials can have different weights even when they have the same volume (take up the same amount of 3-D space). We call this property "heavy for size". The sand is heavy for its size compared with the same volume of water, mineral oil, or organic soil.
Summarize the discussion and recap the investigation
Here is what I think I heard you say about the data we collected...
In your summary, include the two main parts of the data discussion (1) ideas about how to explain the fact that different groups got different results and how to come up with a single weight, and (2) once they have a single weight for 40cc of each material, ideas about how to answer the investigation question.
Here are some of the things we discussed and explored today:
- Volume is the amount of 3-D space an object takes up.
- We measured the weights of equal volumes of sand, organic soil, water, and oil.
- We think we got slightly different weights for each type of material because we used different scales and there may be small differences between the scales.
- Even though we got slightly different measurements, we agreed that 40cc's of water weigh 40g, etc. (read the values from the table).
- We answered the investigation question using the data we collected: equal volumes of different materials do not have the same weights. So some materials are heavier for their size than other materials.
Save the class data chart for Investigation 2.4.