What are some properties of air? (2)
Plan Investigation 15
At the macroscopic scale, air is common and familiar. At the particle scale, it remains such a mystery. In the seemingly still air of a room, countless particles (molecules) of nitrogen, oxygen, water vapor and other gases move faster than the speed of sound, constantly colliding with one another.
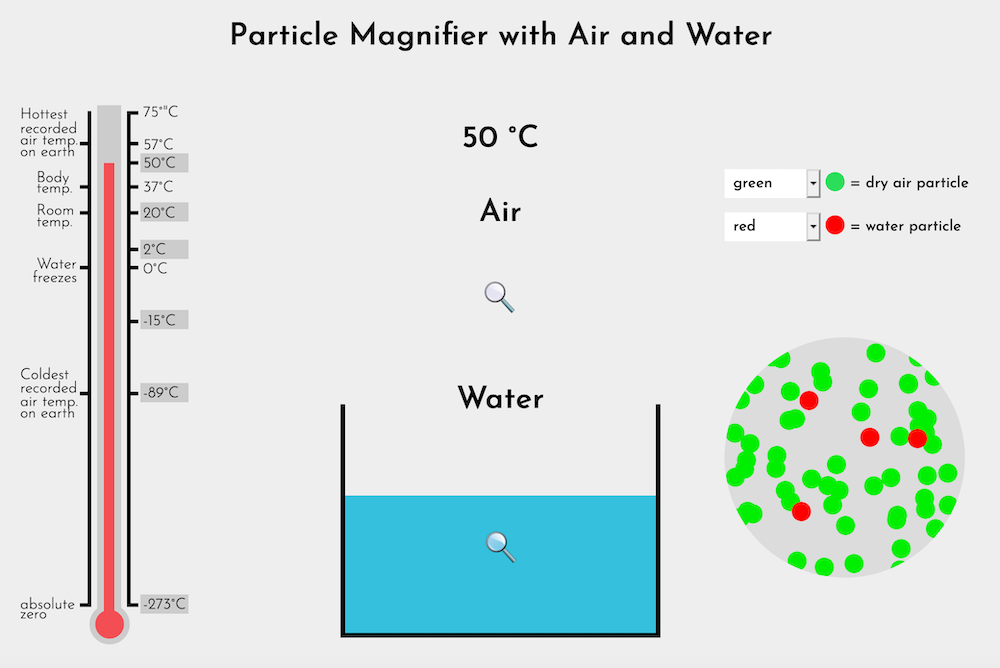
In today's investigation, students discuss two annotated drawings that explain the difference in compressibility between air and water. They use their experience with the Particle Magnifier to reason why water is not compressible. In discussion, they generate ideas about a particle explanation for the compressibility of air. This sets the stage using the Particle Magnifier to look at the particle model of air, a gas.
By the end of this investigation, students will understand that air is composed of particles too small and spread out to see. They will understand that because the particles are spread out, there is space for them to move closer together when air is compressed. Students will also know that air is a mixture of gases.
Learning Goals
- Understand that air is made of particles too small and spread apart to see
- Understand that air is a mixture of different gases, including water vapor
- Understand that the relative compressibility of air and water can be explained in terms of the configuration of their particles
| Sequence of experiences | ||
|---|---|---|
| 1. Discuss annotated drawings | Discussion | 20 Mins |
| 2. Show the Particle Magnifier | All Class | 15 Mins |
| 3. Add to Properties of Air chart | All Class | 10 Mins |
Materials and Preparation
For the class:
- Post the investigation question in a place where all students can see it.
- Annotated Drawing Poster (See Resource Quick Links, also used in Investigation 6)
- 12 copies of each of the two annotated drawings you selected from the previous investigation or Sample Annotated Drawings (See Resource Quick Links)
- Dot Sheet 1 [pdf] and Dot Sheet 2 [pdf] (See Resource Quick Links)
- The Particle Magnifier (Water-Air), using a classroom computer and projector or Smart Board
[Launch Particle Magnifer (Water-Air) in a new window] - Properties of Air chart with the addition column: Particle Explanation; an example is found in Step 2.
Prepare for use in Investigation 16:
- Create bubble mix with the following ingredients. Mix these liquids, cover, and let sit for at least 12 hours prior to Investigation 16.
- 80cc water
- 40cc Joy dishwashing liquid
- 10cc glycerin



