What are some properties of air? (2)
Plan Investigation 15
At the macroscopic scale, air is common and familiar. At the particle scale, it remains such a mystery. In the seemingly still air of a room, countless particles (molecules) of nitrogen, oxygen, water vapor and other gases move faster than the speed of sound, constantly colliding with one another.
In today's investigation, students discuss two annotated drawings that explain the difference in compressibility between air and water. They use their experience with the Particle Magnifier to reason why water is not compressible. In discussion, they generate ideas about a particle explanation for the compressibility of air. This sets the stage using the Particle Magnifier to look at the particle model of air, a gas.
By the end of this investigation, students will understand that air is composed of particles too small and spread out to see. They will understand that because the particles are spread out, there is space for them to move closer together when air is compressed. Students will also know that air is a mixture of gases.
Learning Goals
- Understand that air is made of particles too small and spread apart to see
- Understand that air is a mixture of different gases, including water vapor
- Understand that the relative compressibility of air and water can be explained in terms of the configuration of their particles
| Sequence of experiences | ||
|---|---|---|
| 1. Discuss annotated drawings | Discussion | 20 Mins |
| 2. Show the Particle Magnifier | All Class | 15 Mins |
| 3. Add to Properties of Air chart | All Class | 10 Mins |
Materials and Preparation
For the class:
- Post the investigation question in a place where all students can see it.
- Annotated Drawing Poster (See Resource Quick Links, also used in Investigation 6)
- 12 copies of each of the two annotated drawings you selected from the previous investigation or Sample Annotated Drawings (See Resource Quick Links)
- Dot Sheet 1 [pdf] and Dot Sheet 2 [pdf] (See Resource Quick Links)
- The Particle Magnifier (Water-Air), using a classroom computer and projector or Smart Board
[Launch Particle Magnifer (Water-Air) in a new window] - Properties of Air chart with the addition column: Particle Explanation; an example is found in Step 2.
Prepare for use in Investigation 16:
- Create bubble mix with the following ingredients. Mix these liquids, cover, and let sit for at least 12 hours prior to Investigation 16.
- 80cc water
- 40cc Joy dishwashing liquid
- 10cc glycerin
1. Discussion: comparing annotated drawings
Purpose of the discussion
The purpose of this discussion is for students to develop more refined explanations for the differences they have observed in the compressibility of air and water. The discussion also generates a need for thinking about air on the particle scale. The discussion focuses on the question: Are air and water compressible?
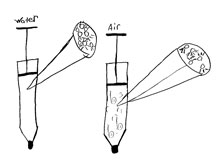
Prepare for all class discussion
Distribute two annotated drawings you selected from Investigation 14 for students to discuss in pairs. Have student pairs briefly review and discuss the two drawings (5 minutes).
Students should use the discussion questions on the Annotated Drawings poster that you have displayed (these are also found on the Annotated Drawings resource at the back of the Science Notebooks).
- What are these drawings trying to explain? (FYI: the reason why air is compressible and water is not)
- Do you see anything in the drawing that you don't understand or would like clarified?
- If you compare the two annotated drawings, how are the explanations the same? How are they different?
Begin the all-class discussion by noting that water is not compressible. Remind students that they used the Particle Magnifier to look at the arrangement of particles in water. You may want to show the class Dot Sheets 1 and 2.
Engage students in the focus question
Why do you think air could be compressed and water could not?
Why do you think the water could not be compressed?
- Water is visible so this means the particles are close together. (Dot Sheet 1 – liquid water)
- Water cannot be visibly compressed because the particles are already close together and can't get any closer.
Next, build on students' ideas about why water can't be compressed to think about why air is compressible. Keep the focus on particles in these explanations.
Why do you think air can be compressed?
- Air is invisible so this means the particles are more spread out than particles of water. (Dot Sheet 2 - air)
- There are wider spaces between the particles of air.
- Air is visibly compressible because there is a lot of space for the particles to be squeezed closer together. This is not true for water, or for any liquid or solid.
Summarize the discussion
Students may not reach a scientifically accurate explanation of compressibility. This presents the need for a model of what is happening at a scale too small to see. The scientist's model of the structure of air in the Particle Magnifier is introduced.
2. Show the Particle Magnifier
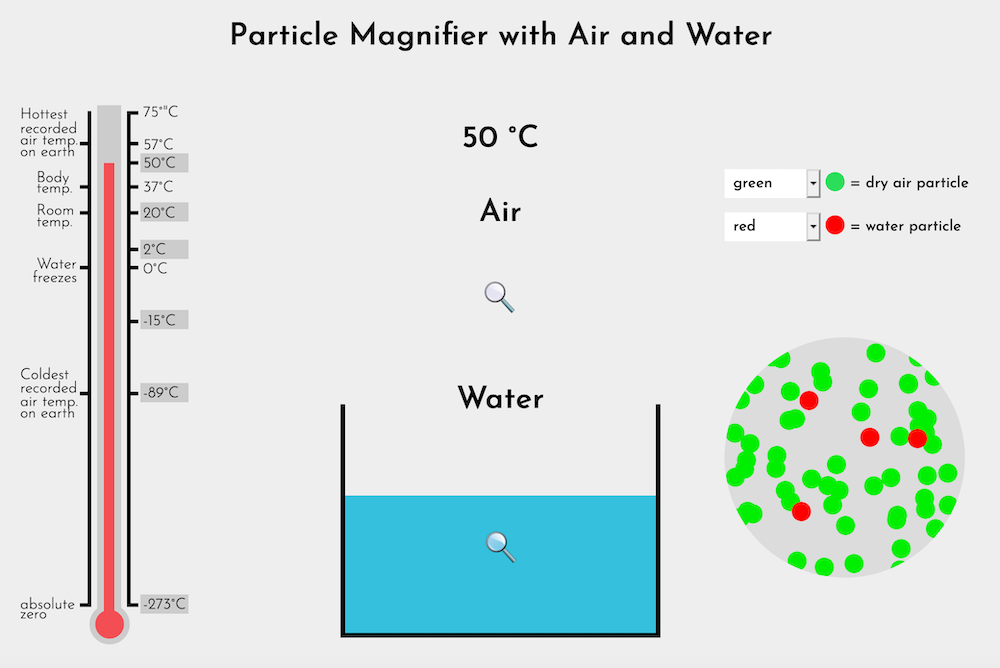
Note: The full version of the Particle Magnifier (Water-Air) allows users to view air and water vapor in addition to the ice / water inside the container. There is no magnifier in the air at -273°C. Nitrogen, oxygen, and all of the elements that compose our atmosphere would be solids before the temperature reached absolute zero.
Unlike the ice-water transformation, there is no emphasis on gradual change in air with temperature change. The key point to highlight is the contrast between air and water.
Remind students that this is an animation that shows what scientists think happens. At each temperature, ask students:
| Temp | Notes | Likely student observations |
|---|---|---|
| +2°C | Show water. This is a reminder to help highlight the difference between water and air. |
|
| +2°C | Show air. |
|
This is the first time students have seen air represented as distinct particles. Remind them of these key points:
- Students have evidence that air has weight, and takes up space, and is therefore matter.
- Scientists have learned that all matter is made of tiny particles too small to see.
- When tiny particles are clumped together, as they are in water, we can see the matter. (Dot Sheet 1)
- When those particles are spread apart, as they are in air, or in salt that has been dissolved in water, we cannot see the matter. (Dot Sheet 2)
Move back and forth between water and air, at different temperatures, highlighting the difference in the distance between particles.
What are your ideas now about why air is compressible and water isn't?
- There is more space between the air particles so there's room for them to move together when air is compressed (squeezed).
3. Add to Properties of Air chart
Refer to the chart. Add air is compressible to the properties list. Point out that there is now a third column in the chart called Particle Explanation.
Model a response by completing the first row yourself.
The first property is air is invisible. A particle explanation would be air particles are too tiny and too spread out to see. We used Dot Sheet 1 to help us understand this.
Complete the Particle Explanation column for the properties listed so far.
| Properties | Evidence or Reasoning | Particle Explanation |
|---|---|---|
| Air is invisible. | I can see through it. | Air particles are too tiny and too spread apart to see. |
| Air has weight. | I saw the filled balloons tip the balance in the last science class. | Air is matter and each air particle has a tiny bit of weight. |
| Air takes up space. | Air made the plunger of the syringe move. | Air is matter and each air particle takes up a tiny bit of space |
| Air has no odor or taste. | Personal observations. | |
| Air is compressible. | I squeezed the syringes from 12ml to 6ml and when I stopped squeezing, the plunger returned to 12ml. | Air particles have a lot a space between them so they can be squeezed together. |
What's in air?
Explain that although all of the air particles looked the same in the Particle Magnifier, air is actually a mixture of different gases.
Just two gases, nitrogen and oxygen, make up more than 99% of the volume of dry air at ground level. Water vapor in the air varies from a trace to 4%. The amount of water vapor is limited by the temperature of Earth's atmosphere. More than a dozen gases exist in air in tiny amounts, (these are called trace gases) including neon, helium, methane, hydrogen, ozone, carbon monoxide, and sulfur dioxide.
Recap the investigation
Review the key ideas of the investigation:
- Like water, air is made of unimaginably tiny particles.
- Air particles are too small and too spread apart to see.
- The greater space between air particles explains why air is compressible while water is not.
- Air is a mixture of different gases, including water vapor.



