What are some properties of air? (1)
Plan Investigation 14

Having established that air is matter in the last lesson, this investigation explores some properties of air, a gas. If you have added air to the tires of a car or a bicycle, you have had some experience with compressed air. It takes some effort, but it's possible to squeeze a large volume of air into a significantly smaller space. This is not true for liquids or solids, which are essentially incompressible. What is it about air, and gases in general, that allows it to be compressed? In comparison with solids and liquids, the individual particles (atoms or molecules) of any gas are quite far apart from one another, with nothing but a vacuum in between them. Therefore, when pressure is applied to a gas, the particles can be squeezed closer together.
Formative Assessment
Available online at inquiryproject.terc.edu
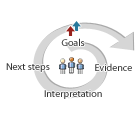
Can students envision what might happen at the microscopic level to explain why air is more compressible than water?
Look for evidence of students explanations in their annotated notebook entries on the page Explaining the difference in compressibility of water and air.
As you interpret student work, remember, students have not yet viewed air with the Particle Magnifier. They are basing their ideas on first–hand experience compressing air and water and using the Particle Magnifier to explore particles of water and ice.
Does the drawing show that:
- Air is more compressible than water.
- Both water and air are made of particles.
- There is more space between air particles so they can be pushed closer together.
A next step might be to ask students if they think ice would be compressible or not.
Today students continue to explore the properties of air. Students compare the compressibility of air and water and then develop an annotated drawing that responds to the question, "How do you explain the difference in the compressibility of air and water?"
By the end of this investigation students will understand that, in contrast with liquids and solids, air is observably compressible and will make a drawing to explain their observations.
Learning Goals
- Understand that air is compressible
| Sequence of experiences | ||
|---|---|---|
| 1. Ask the question | All Class | 10 Mins |
| 2. Explore compressibility | Individual | 15 Mins |
| 3. Create an annotated drawing | Individual | 20 Mins |
Materials and Preparation
Preparation:
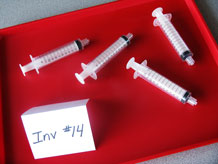
- Fill 4 12cc syringes with water, eliminate air bubbles, and install caps to the open ends using finger pressure. Do not use a lot of force to tighten the caps.
- Fill 4 12cc syringes with air and install caps to the open ends using finger pressure.
For the class:
- Post the investigation question in a place where all students can see it.
- Annotated Drawing Poster (See Resource Quick Links, also used in Investigation 6)
- Make a class chart titled, "Properties of Air"; an example is found in Step 1.
- 2 1gal buckets
- 4 12cc syringes filled with water, with caps
- 4 12cc syringes filled with air, with caps
For each group:
- 4 12cc syringes without caps
- 12 copies of each of the two annotated drawings you select from today's class
1. Ask the question
Now that air has been established as matter, students begin a list of air's properties. Introduce the investigation question:
What are some properties of air?
Record properties of air that students can identify. As they mention a property ask for evidence or reasoning that supports their claim.
| Properties | Evidence or Reasoning |
|---|---|
| Air is invisible. | I can see through it. |
| Air has weight. | I saw the filled balloons tip the balance in the last science class. |
| Air takes up space. | Air made the plunger of the syringe move. |
| Clear air has no odor or taste. | Personal observations. |
Save the list of properties; students will add properties as they continue their investigation of air.
Introduce the term compressible - able to be squeezed or pressed into a smaller size or volume. One property of a rock is that it is not visibly compressible. One property of a sponge is that it is visibly compressible. Explain to students they'll be exploring the compressibility of air and how it compares with the compressibility of water.
2. Explore compressibility
Predict
Have students predict and explain their reasoning in their Science Notebooks, [Predicting the compressibility of water and air].
Air in a single syringe

Distribute a 12cc syringe to each student. Direct students to:
- Pull the plunger back to the 12cc mark.
- Cover the small opening in the syringe barrel with the palm of one hand.
- Push the plunger.
- Consider these questions:
- Does the plunger move?
- What happens when you release the plunger?
Students only need a minute or two to explore the compressibility of air with the syringes. Collect the syringes when they are finished.
Note: As students explore the compressibility of air, they may become interested in whether or not the syringe plunger returns to the exact same spot once it has been released. It may not, due to friction between the rubber seal and the plastic barrel of the syringe, or because a tiny bit of air leaked out between the syringe and the palm of the hand. Neither of these possibilities should distract from the big idea that air can be squeezed into a smaller space, and will expand again once the plunger of the syringe is released.
Water and air in a syringe
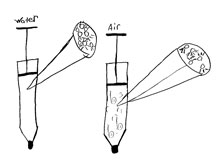
How does the compressibility of water compare with the compressibility of air?

Move from group to group with two plastic buckets each holding two capped water-filled syringes and two capped air-filled syringes. Under your supervision, give four students at a time an opportunity to compress water and air in syringes. Be sure students point the capped ends of their syringes into one of the plastic buckets before they apply pressure to the plungers. In the unlikely event that the pressure causes a cap to release, the water and cap will end up in the plastic bucket.
Provide a few minutes for students to record observations on the [Observing the compressibility of water and air] page in their Science Notebooks.
Note: Liquids and solids are compressible, but only to a tiny degree in comparison with air or gases in general.
Is there another property of air we can now add to the list?
- Air can be squeezed into a smaller space; it is compressible.
Does this compressibility change our finding that air takes up space?
- No. Air still takes up space, but the amount of space it takes up can change when it is squeezed or compressed in a closed system. (Sealing the opening of the syringe changes it temporarily into a small closed system.) Air can't be squeezed so much that it takes up no room at all.
Letter from the Engineer
When you compressed air in the syringe, maybe you noticed that it pushed back with a little bounce. Air acts somewhat like a spring. You can compress it, or squeeze it into a smaller volume. When you push on the plunger you can feel the air pushing back. When you stop pushing, the air inside the syringe will return to its original size.
There are different ways to compress air. One way is to squeeze a certain volume of air into a smaller space. That’s what you did with the syringe. Another way is to use a pump, like a bicycle pump, to squeeze extra air into something that is already full of air. That’s what you do when you pump air into a basketball, soccer ball, or bicycle tire. Why is it possible to add more air to something that is already full of air? Only because air is easily compressible — the particles can be squeezed closer together. (Remember how different it felt when you tried to compress water in the syringe?)
The bouncy feeling you notice when you compress air can be very useful. When you dribble a basketball, the part of the ball that hits the floor gets pressed into the ball a little bit. It’s a little like pushing the handle of the syringe or squeezing a spring. The air inside the ball gets squeezed, and it springs back, just like the handle of the syringe did. This “springing back” makes the ball bounces back up to you. Maybe you have tried to dribble a basketball or kick a soccer ball that did not have much air in it. If you have, you know the ball does not have the same kind of springiness or bounce.
3. Create an annotated drawing
Use the Annotated Drawing poster to review the purpose and important characteristics of an annotated drawing. Students may want to review the Annotated Drawings at the back of the Science Notebook. They should make their drawings on the page [Explaining the compressibility of water and air] in their Science Notebooks. Their drawings should answer the questions below.
- How do you explain the difference in the compressibility of air and water?
- What would you see at the particle level to explain what is going on?
Note: You will need to select two drawings that offer different explanations for students to review in the next investigation. Try to find a drawing that portrays water as made of particles that are packed together (making it visible) and air as made of particles that are spread apart (making air invisible) and select it for the class to review. Select a second drawing that portrays an alternate explanation of the composition of water and air. Alternately, you can choose to use the Sample Annotated Drawings found in the References.
Recap the investigation
Explain that you are going to select two annotated drawings based on different ideas about why air is compressible and water is not. The class will discuss those drawings at the start of the next investigation.