How do oil and water compare?
Plan Investigation 3.3

Mineral oil and water — they are alike in many ways. But their differences may tell us more about the nature of Earth's materials. In this investigation, students explore some differences between oil and water as they make their way toward an understanding of density.
Formative Assessment
Available online at inquiryproject.terc.edu
First students compare some properties of oil and water by sight, then they measure volumes of oil and water at three different weights. They find a relationship between the weights and volumes and consider whether the relationship holds true for all weights of these materials.
By the end of this investigation students will understand that oil will always have more volume than water when their weights are equal. With this they have the beginnings of an understanding of density as the amount of space a material takes up per unit of weight.
Learning Goals
- Observe some differences between oil and water
- Compare the amount of space a gram of water and oil take up
- The volume of one gram of a particular liquid is a property of that material
| Sequence of experiences | ||
|---|---|---|
| 1. Ask the question | All Class | 10 Mins |
| 2. Explore oil and water | Small Groups | 10 Mins |
| 3. Share weight and volume data | All Class | 15 Mins |
| 4. Make meaning | Discussion | 10 Mins |
Materials and Preparation
For the class:
- Post the investigation question in a place where all students can see it.
- Make an overhead transparency of the notebook page titled "Observations about oil, water, weight, and volume," or copy it onto a class chart for everyone to see (optional).
- 1 classroom weight line (see kit for instructions)
- Blue food coloring

For each tray:
Different groups work with different-sized samples; you will prepare 3 sets of trays, one with 20g of oil, another with 40g, and a third with 80g.
- 1 index card labeled "20 grams" or "40 grams" or "80 grams"
- 1 capped 150cc container with exactly 20 or 40 or 80 grams of mineral oil *
- 1 empty 150cc covered container *
- 1 20oz cup approximately half filled with blue-tinted water
- 2 paper towels
- 1 pipette
- 1 digital scale
- 1 fine tip permanent marker
Do students understand that when the weights of oil and water are equal, the oil will always occupy more space?
Listen for evidence of understanding as the class discusses their findings. How would you interpret this student’s statement?
- Will the volume of the oil always be greater?
- What is her claim?
- What evidence does she use to back up her claim?
A next step might be a question, “What if we had 30 grams of oil and 30 grams of water, how would the volumes compare? Explain the reasons for your answer.”
1. Ask the question
Review with the class what we've learned about liquids so far in this unit. We know that liquids flow and take the shape of their containers. We know that we can measure their volume "by eye," or by using cubic centimeters, or with a measured cup. Point out that this is true for all liquids.
But are all liquids alike? Take mineral oil and water, for example. Would you use water to grease a pan? Would you use oil to put out a fire?
Share today’s investigation question:
How do oil and water compare?”
Begin a discussion of what students already know about the properties of oil and water:
- What are some properties of water that you have noticed?
- e.g., water is clear, "wet," "thin," and "splashy"
- What are some properties of oil that you have noticed?
- e.g., oil is "sticky," "slippery," "thick," and pours slowly

Remind students that they know something else about oil and water. Ask them to look back at the data they collected when they compared the volumes of 40 grams of water, oil, sand, and soil (Heavy for Size, Investigation 2.1, Observations of Weights and Volumes).
- How did the volumes of oil and water compare?
- the oil had a greater volume
- What if, instead of 40 grams of oil and water in the container, you had only 20 grams of each. Try to picture it. How would the volumes compare? Would there be a greater volume of oil or a greater volume of water? Or would they be the same?
- What if you had 80 grams each of oil and water? How do you predict the volumes would compare?
As students share their ideas, let them know they are about to find out if their predictions are correct.
2. Explore oil and water


As you distribute a tray of materials to each group, explain that groups will get 20 grams or 40 grams or 80 grams of oil. They will measure out equal amounts of water in a moment. At the end of the investigation, they will share their results and compare them.
Students fill the empty container with their assigned weight of water — 20 grams, 40 grams, or 80 grams. You may need to remind them to allow for the weight of the container (26g). Students then double-check that the weight of their two samples is the same; if it's not, they can add or remove some water using the pipette.
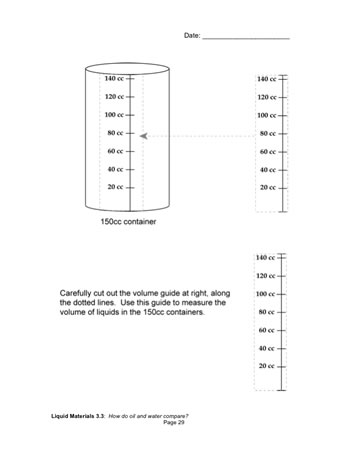
When they have equal weights of oil and water, students measure the volumes of the two samples using a measuring strip that they cut out of their notebooks. They record the volumes on the containers and make labeled drawings of the containers in their notebooks [How do oil and water compare?].
As students finish up their measurements, ask them to compare the way oil and water move by holding a container upright in each hand and gently swirling the liquids — no shaking or splashing! Have them record and share their observations.
- What property of oil or water do you think explains the difference in the way these two liquids move?
No oil spills! Though the containers of oil are capped and taped shut, students should keep them top-up at all times to prevent oil from leaking out. This is especially true when they are investigating the way the liquids move — no shaking!
3. Share weight and volume data

Ask the students to come to the class weight line with their notebooks and their containers of oil and water. Have each group place their containers at the appropriate point on the line (there will be duplicates).
- What do you notice?
Students may struggle a little to see the weights and volumes, but there should be consensus that the volumes are increasing as the weights increase. They may or may not be able to tell much about how the volumes of the oil and water samples compare.
Chart data (optional)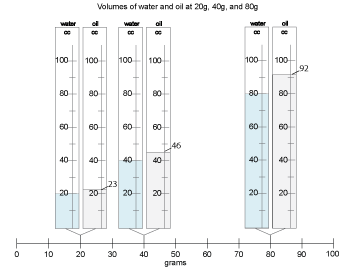
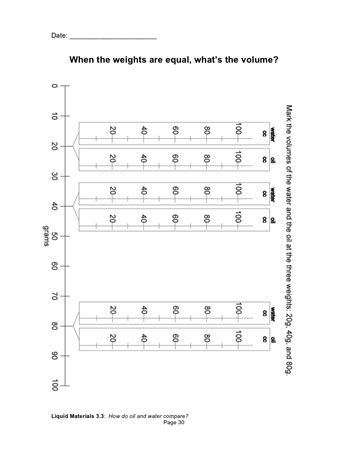
Let's look at the data another way. Let's take the weight and volume data from your notebooks and put it on a class chart.
Have students open their notebooks [When weights are equal, what’s the volume?]. Point out that this is the same chart as the class chart, only sideways.
- How does this chart work?
- Where should we put the volume data for the 20 grams of water?
Record data for all the samples on the chart, resolving any disputed findings as you go along. Have students record the data in their notebooks. The chart will look much like this.
4. Make meaning
Purpose of the discussion
The purpose of the discussion is for students to use the data they've collected to make and support claims about the investigation question. Return to the investigation question for discussion.
Engage students in the focus question
Now we can see the data more clearly. What claims can we make that the data support?
How do oil and water compare?
- Look carefully at the differences in weights and in volumes. What do you notice?
- If the weights are the same, oil has greater volume than water in every case.
- The heavier the samples, the greater the difference between the volumes.
- As the weight doubles, the volume difference doubles.
Supporting questions
- What do you think the volume difference for oil and water would be if you had 160 grams of each liquid?
- How do you think the volumes would compare if you had 5 grams of each? Or just 1 gram of each? Or if the samples weighed tons and tons
- If instead of starting with equal weights you started with equal volumes: how do you think the weights would compare?
- If the volumes are the same, oil will weigh less than water.
- What property of oil or water do you think explains why equal weights of oil and water have different volumes?
The class knows this property from earlier investigations as "heavy for size." We can also say "heavy for volume." Water is heavy for its volume compared with oil, just as sand is heavy for its volume compared with soil.
Summarize the discussion and recap the investigation
Use the same language that students have used to summarize the discussion. Include student comments that address claims and evidence about how oil and water compare.
As you recap the investigation, be sure there is understanding of these two points:
- If the weights of oil and water are equal, oil will always have a greater volume than water.
- The amount of space a liquid takes up compared to its weight is the property we call "heavy for size."
Not too small to matter. Heaviness for size is a property of all materials, independent of sample size. Although it is not very obvious, a single gram of oil has a greater volume than a single gram of water, and 0.1 grams of oil will have greater volume than 0.1 grams of water. If students doubt this, ask if they are saying we might not notice the volume difference any more, or if they think the difference really ends once we reach 1 gram. While our senses are very important in helping us learn about the world, there are times when our senses — or even scientific instruments — cannot detect something, and we have to rely on our understanding. Does it make sense that the pattern we see would suddenly stop being true at 1 gram? No, there is no logical reason for that, so we must rely on our understanding.