What changes and what stays the same as ice melts?
Plan Investigation 12

Today students complete their investigation of ice. They start by checking the bottles to answer whether weight and volume change as ice melts. At this point students should have substantial evidence for answering the question, What changes and what stays the same as ice melts? at the visible level. To learn about what is happening at a very much smaller scale, the particle level, students observe the Particle Magnifier, a computer model that introduces scientists' understanding that all matter is made of tiny particles (atoms or molecules). As the ice is warmed, students see the motion of individual particles increase to the point where the bonds holding the particles locked in position in the solid begin to break and reform. This allows the particles to slide past and collide with one another as the ice is transformed to water.
By the end of this session students will understand that, as ice melts, the weight of a sample remains the same, the volume decreases, and the kind of matter - water - remains the same. They will have been introduced to the particle model of matter and considered how the model helps to explain the transformation of a solid to a liquid.
Learning Goals
- Understand that liquid water and ice are the same kind of matter
- Become familiar with the particle model of matter to explain transformations of water
| Sequence of experiences | ||
|---|---|---|
| 1. Explore bottles | Pairs | 10 Mins |
| 2. Use data | All Class | 5 Mins |
| 3. Introduce Particle Magnifier (Water) | All Class | 15 Mins |
| 4. Make meaning | All Class | 15 Mins |
Materials and Preparation
Preparation:
- Read The Particle Magnifier (See Resource Quick Links)
- Explore the Particle Magnifier (Water)
[Launch Particle Magnifer (Water) in a new window]
For the class:
- Post the investigation question in a place where all students can see it.
- Post the class chart, "Comparing Ice and Water"; created in Investigation 10.
- Post the class table, "Transforming Water to Ice and Ice to Water"; created in Investigation 11.
- The Particle Magnifier (Water), using a classroom computer and projector or Smart Board.
For each group:
- 1 digital scale
- 2 plastic bottles of water, from the last investigation
1. Explore bottles

The class will finish their investigations of ice today. After they check the weight and volume of the melted ice, they will return to the investigation question:
What changes and what stays the same as ice melts?
Distribute materials. Give each group the same scale it has been using to weigh the water and ice in the previous classes.
Point out that there is no condensation on the containers. This should reinforce the fact that water does not seep through the plastic, and that temperature plays a role in condensation. Students:
- Measure and record the weight on the page [What happens to weight and volume when water freezes and ice melts?] in the Science Notebooks.
- Check the volume by observing the level of the water and record the observation (did the volume increase, decrease, or stay the same?) on the same page.
- Respond to the questions on the page [What changes and what stays the same as ice melts?] in their Science Notebooks.
- Enter the weight and volume data in the last two columns of the class data table.
| Pair | Weight before Freezing (grams) | Weight after Freezing (grams) | Volume after Freezing (Notes) | Weight after Melting (grams) | Volume after Melting (Notes) |
|---|---|---|---|---|---|
| 1 | |||||
| 2 | |||||
| 3 | |||||
| etc. |
2. Share data/Argue from evidence
Use the data from the class chart, Comparing Ice and Water and/or the class data table, Transforming Water to Ice and Ice to Water to address the following:
Some possible student arguments:
| Yes, ice and water are the same kind of matter | No, ice and water are not the same kind of matter |
|---|---|
|
|
Explain that you are going to introduce a scientific model that may help them think in a new way about the matter that makes up ice and water.
3. Introduce Particle Magnifier (Water)
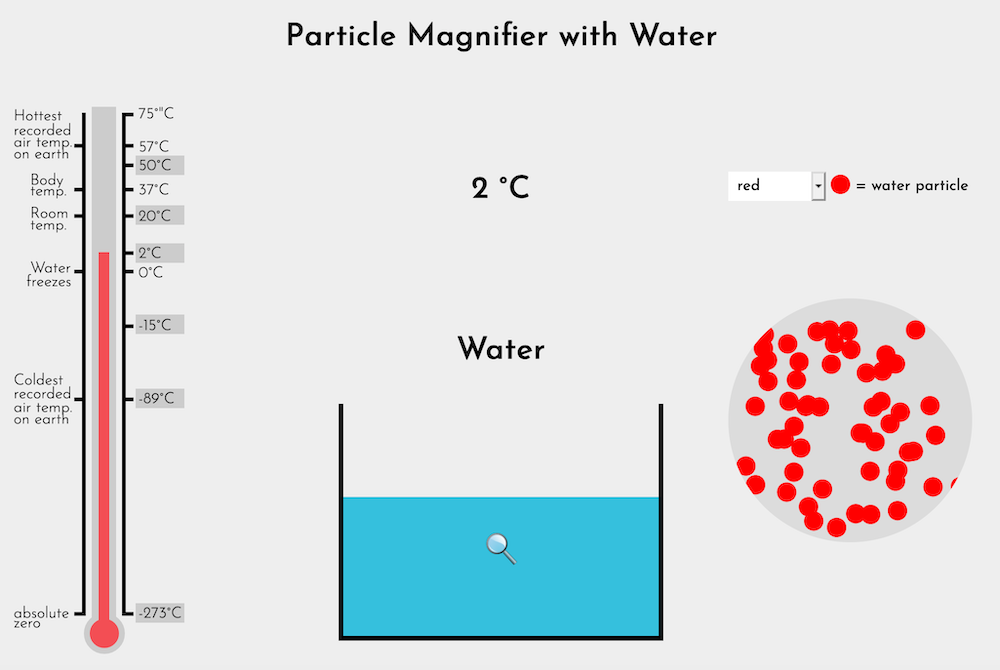
Explain that scientists believe all matter, including ice and water, is made of very tiny particles. Single particles of matter are much too small to see even with the most powerful microscopes available. Once enough of the particles clump together, we see them with our eyes as water, or ice, or salt, or other materials. The Particle Magnifier (Water) represents what scientists imagine they might see if they could see individual particles of ice or water at various temperatures.
Start by selecting Absolute zero on the thermometer of the Particle Magnifier (Water) and show the ice/water at increasing temperatures. At each temperature, provide a few words of context (see Notes, below) and ask:
| Temp | Notes | Likely student observations |
|---|---|---|
| Absolute zero | No heat energy is present. |
|
| -89°C | The ice is still extremely cold, but heat energy is causing the particles to vibrate. |
|
| -15°C | The ice is warmer, but is still quite frozen. |
|
| 2°C | Enough heat energy has been added to transform ice to water. |
|
| 20°C | The water is at room temperature. |
|
| Temp | Notes | Likely student observations |
|---|---|---|
| 50°C | The water is now too hot to touch, but it is not boiling. |
|
How does the Particle Magnifier explain some of the differences between solids and liquids?
- Solid materials have a shape because the particles are locked together in a lattice or grid.
- Liquids flow because the particles have more heat energy, have broken away from one another, and can slide past and/or bump one another.
Optional: The Science Notebook page [Particle Magnifier ice and water particles] is there for you to assign if time permits.
4. Make meaning
Purpose of the discussion
The purpose of the discussion is for students to clarify their understanding of transformation of solid ice to liquid water at the particle level. Focus the discussion on the investigation question: What changes and what stays the same as ice melts?
Engage students in the focus question
Imagine a tray of ice cubes. Imagine those cubes melting. If we could zoom in and see the ice melt at the particle level - like we did with the Particle Magnifier - what do you think would change and what would stay the same as ice melts?
- The number, size and shape of individual particles stay the same when ice melts.
- For both ice and water, the warmer the temperature, the more the particles move.
- In ice, the particles are arranged in a pattern, even when they are warmed up and vibrating (jiggling but still in place).
- In water, individual particles slide around and collide, and are not arranged in a pattern.
Revisit the Particle Magnifier (Water)
What connections do you notice between the visible evidence and changes at the microscopic level?
| Visible Level | Particle Level |
|---|---|
| Solid water (ice) keeps its shape and liquid water doesn't. | Particles in ice are held in a shape. Particles in water aren't locked in place. They slide past and collide with one another. |
| At the visible level, reasoning tells us that water remains the same - ice is formed from water and returns to water when it melts. | Particles in the model remain the same. Particle motion and arrangement change. |
Summarize the discussion and recap the investigation
When a sample of water is transformed to ice and back to water, the weight does not change. When water freezes, the volume increases but returns to the original volume when the ice melts. The particle model of matter explains these transformations of water.
Note: Materials typically contract when they cool and change from liquid to solid. Water is an exception: it expands when it freezes and transforms to ice.









