What changes and what stays the same as ice melts?
3. Introduce Particle Magnifier (Water)
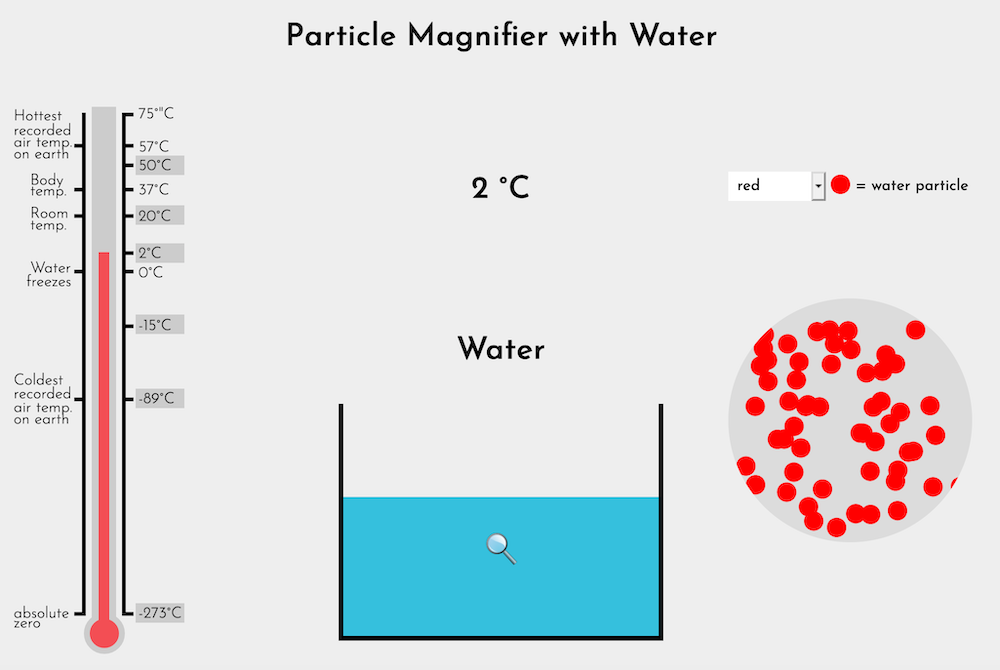
Explain that scientists believe all matter, including ice and water, is made of very tiny particles. Single particles of matter are much too small to see even with the most powerful microscopes available. Once enough of the particles clump together, we see them with our eyes as water, or ice, or salt, or other materials. The Particle Magnifier (Water) represents what scientists imagine they might see if they could see individual particles of ice or water at various temperatures.
Start by selecting Absolute zero on the thermometer of the Particle Magnifier (Water) and show the ice/water at increasing temperatures. At each temperature, provide a few words of context (see Notes, below) and ask:
| Temp | Notes | Likely student observations |
|---|---|---|
| Absolute zero | No heat energy is present. |
|
| -89°C | The ice is still extremely cold, but heat energy is causing the particles to vibrate. |
|
| -15°C | The ice is warmer, but is still quite frozen. |
|
| 2°C | Enough heat energy has been added to transform ice to water. |
|
| 20°C | The water is at room temperature. |
|
| Temp | Notes | Likely student observations |
|---|---|---|
| 50°C | The water is now too hot to touch, but it is not boiling. |
|
How does the Particle Magnifier explain some of the differences between solids and liquids?
- Solid materials have a shape because the particles are locked together.
- Liquids flow because the particles have more heat energy, have broken away from one another, and can slide past and/or bump one another.
Optional: The Science Notebook page [Particle Magnifier ice and water particles] is there for you to assign if time permits.



