What are some properties of air? (1)
Plan Investigation 14

If you have added air to the tires of a car or a bicycle, you have had some experience with compressed air. It takes some effort, but it's possible to squeeze a large volume of air into a significantly smaller space. This is not true for liquids or solids, which are essentially incompressible. What is it about air, and gases in general, that allows it to be compressed? In comparison with solids and liquids, the individual particles (atoms or molecules) of any gas are quite far apart from one another, with nothing but a vacuum in between them. Therefore, when pressure is applied to a gas, the particles can be squeezed closer together.
Formative Assessment
Available online at inquiryproject.terc.edu
Can students envision what might happen at the microscopic level to explain why air is more compressible than water?
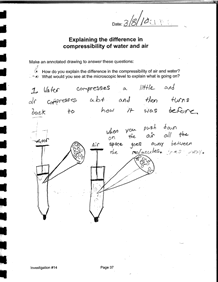
Look for evidence of students explanations in their annotated notebook entries on the page Explaining the difference in compressibility of water and air.
As you interpret student work, remember, students have not yet viewed air with the Particle Magnifier. They are basing their ideas on first–hand experience compressing air and water and using the Particle Magnifier to explore particles of water and ice.
Does the drawing show that:
- Air is more compressible than water.
- Both water and air are made of particles.
- There is more space between air particles so they can be pushed closer together.
A next step might be to ask students if they think ice would be compressible or not.
Today students continue to explore the properties of air. Students compare the compressibility of air and water and then develop an annotated drawing that responds to the question, "How do you explain the difference in the compressibility of air and water?"
By the end of this investigation students will understand that, in contrast with liquids and solids, air is observably compressible.
Learning Goals
- Understand that air is compressible
| Sequence of experiences | ||
|---|---|---|
| 1. Ask the question | All Class | 10 Mins |
| 2. Explore compressibility | Individual | 15 Mins |
| 3. Create an annotated drawing | Individual | 20 Mins |
Materials and Preparation
Preparation:
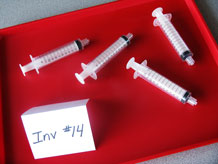
- Fill 4 12cc syringes with water, eliminate air bubbles, and install caps to the open ends using finger pressure. Do not use a lot of force to tighten the caps.
- Fill 4 12cc syringes with air and install caps to the open ends using finger pressure.
For the class:
- Post the investigation question in a place where all students can see it.
- Annotated Drawing Poster (See Resource Quick Links, also used in Investigation 6)
- Make a class chart titled, "Properties of Air"; an example is found in Step 1.
- 2 1gal buckets
- 4 12cc syringes filled with water, with caps
- 4 12cc syringes filled with air, with caps
For each group:
- 4 12cc syringes without caps
- 12 copies of each of the two annotated drawings you select from today's class