What are some properties of air? (3)
4. Discuss annotated drawings
Purpose of the discussion
The purpose of the discussion is for students will understand that air expands when it is warmed and contracts when it is cooled and these phenomena can be explained in terms of the motion of particles.
Engage students in the focus question
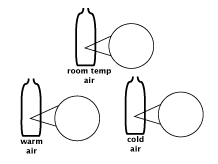
How do warm air, cool air, and room temperature air differ?
Make a large sketch similar to the one in the Science Notebooks.
- Ask students how you should draw the soap film in all three bottles.
- Draw particles of room temperature air in the magnifier circle.
- Ask students to recommend how you should draw the particles in the magnifier circle for warmer air. Encourage them to refer to their own ideas that they represented in their drawings.
What is the same about the particles in room temperature and warmed air?
- They are the same size — the particles stay the same.
What is different about the particles?
- They are farther apart (are more spread out, there is more space between them).
Why did the bubble form?
- When the temperature is higher, the particles move faster and they bump into each other more often and move farther apart making the air take up more space.
- ==> The only space the air could move to was outside the bottle so the air pushed up on the soap film.
Draw the particles in the magnifier circle for the cooler air.
Why did the soap film move down into the bottle when the air was cooled?
- When the air is cooler, the particles move more slowly and they moved closer together. When there was less space between the particles, the air in the closed system took up less space in the bottle so the soap film moved down.
If you put a magnifier on air near the top of the bottle and one on the air that's near the bottom, how do you think the particles and spaces between them would compare?
- They would look the same in both places because air particles are evenly distributed in the bottle.
It is not unusual for students to think that "hot air rises" so there will be more air particles near or in the bubble and few or none at the bottom of the bottle. You may want to explain that if we were able to see the particles, we would find they are quite evenly distributed throughout the bottle.
- There will be many more air particles near the top than at the bottom of the bottle (a frequently held but incorrect idea)
Summarize the discussion and recap the investigation
To summarize the discussion, complete the properties of air chart and review the key ideas for today



